| Home > About Graphite |
|
|
|
Graphite Characteristics |
|||
 |
"Kokuen" is Japanese for black lead or graphite which has a structure similar to sumi ink, charcoal, and carbon black. The difference in crystal formation is what gives it different characteristics from its brother the diamond. The 4 characteristics of graphite are its superior lubricancy, conductivity, fireproofness, and acid and alkaline proof qualitics. Our job is to refine it in such a way as to achieve 100% of its potential. Some examples of its lubricating qualities are found in its use in pencil lead, bullet train pantographs, and vehicle brake pads. Without graphite, the manufacture of aluminum foil, and engine crank shafts would be impossible. Its conductive powers are put to work in battery compounds television picture tube paint, and in heat seal connectors (used as circuits to transmit signals which in turn activate liquid crystals) in electronic schedule books, calculators, and handy phone. Graphite is not a highly visible material but it is vital for industry, nevertheless.
We are the only consistent manufacturer of graphite products in the world. The more "high-tech" industry the more graphite will be in demand. Industry the world over is eager for graphite. |
||
 |
|||
|
|||
| Fig.1 Crystal structure of graphite 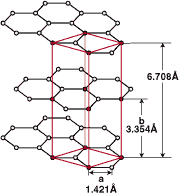 |
The crystal structure of graphite consists of carbon atoms. The carbon atoms within a graphite layer plane from a regular hexagonal network with a C-C distance of 1.421Å. As described by the figure at left the layer planes are stacked parallel to each other at 3.354Å apart. One alternative lattice, with stacking sequence ABAB-,has a hexagonal unit cell (Hexagonal System), as shown in the figure Another alternating (ABCABC-) stacking sequence results in a rhombohedral unit cell (Rhombohedral System). It is also evident from the diagram that conductivity and lubrication is due to the perfect layer structure. |
||
| Copyright 2000 Nippon Graphite Industries, ltd. All rights reserved. |
|